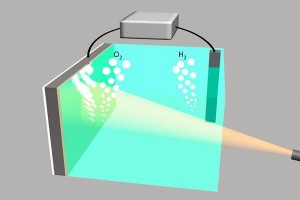
This image shows two electrodes connected via an external voltage source splitting water into oxygen (O2) and hydrogen (H2). The illuminated silicon electrode (left) uses light energy to assist in the water-splitting process and is protected from the surrounding electrolyte by a 2-nm film of nickel.
You can spit water but you can also split water. The splitting of water is important because when you split H2O you get two molecules of hydrogen and one molecule of oxygen. Both are important and hydrogen can be used as a fuel. Unlike solar cells they don’t require sunshine and can be used in places not considered to be sunny enough to efficiently generate energy from solar cells. There have been lots of fuel cells based upon splitting up water and you can even reverse the process to generate clean water from hydrogen and oxygen. The group at Stanford University have used silicon a material also used in solar cells. They coated the electrodes with nickel to help improve the process and lower the cost.
