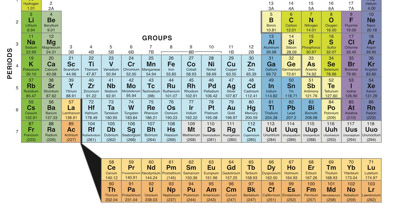
Chemists swear by it, but for the rest of us the periodic table is like a bunch of stuff organized more like your closet at home, with things stuffed into places just because they fit.
In fact, there is a method: the rows (across) are called “periods,” the columns (up-down) are called “groups.”
Right now there are 118 different elements, most of them are natural and a few are man- made. The first element on the periodic table is hydrogen (H), which has an atomic weight of 1, and the last element is something called ununoctium, which has an atomic weight of 118. Only the first 94 elements are believed to occur naturally on Earth. The rest have been created synthetically by scientists.
Atomic weight is the mass of the element, the weight is a total of the protons, neutrons and electrons. It is listed as an average since there is a bit of variation due to things called isotopes. Isotopes have a different number of neutrons, so there is carbon-12 but also carbon-13 and carbon-14. Carbon-14 is an isotope but pretty stable—the amount of carbon-14 in a sample can be used to figure out how old a biological sample is.
Groups are considered the most important way in which we classify elements—group 4A includes things like carbon, silicon, germanium and lead, all of which form stable compounds. They all have five electrons in their outermost shell, allowing them to form a lot of different compounds because the electrons can form bonds with different kinds of atoms. Elements in the same group (that is, the same column) generally have similar chemical properties.