Nanooze Blog
Fixing broken neurons
Spinal injuries can be devastating with the loss of movement in arms and legs. The primary problem is damage to neurons, those cells that transmit signals to and from the brain. There have been many attempts to fix neurons. Scientists at MIT have developed a new synthetic nanomaterial that is like rubber but able to transmit electrical and optical signals. Some day this kind of material could fix connections between tissues and has the mechanical properties that would allow it to be flexible. There are a lot of challenges including making sure that the body accepts this material and doesn’t try to reject it
Slick!
Nature provides a lot of inspiration for making things on the nanoscale. We have evolution to help get the design right and then if we are smart enough we can go into the lab figure out how it works and copy it. Things like gecko feet have been used to develop new types of adhesives (see here). Now researchers at Penn State University have looked at how the carnivorous pitcher plant produces a super-slippery surface that makes ants (and other insects) slide on the plant’s leaf and become……dinner. These super-slippery surfaces can be mimicked using special types of Teflon that these scientists create in the lab. What can we use this super-slippery stuff for? Things like medical devices or even surfaces that we don’t want to ice up, like the wings of a plane. For more information go here.
A nano super hero
Scientists come in all shapes, sizes and colors. One of the super heros of nanotechnology died last week. Mildred Dresselhaus. Who? Dresselhaus was one of the pioneers in the discovery of carbon nanotubes and predicted their existence long before anyone even saw one. Carb on nanotubes are nanometer-sized tubes consisting entirely of carbon. Her contributions to science are many and she is among the most recognized scientists in the field. Dresselhaus worked on graphene and the field of thermoelectrics which has a number of different important applications. How important was her contributions to science and also the promotion of women in science? In 2014 she was awarded the Presidential Medal of Freedom! An award given that year to individuals as diverse as Meryl Streep (actress), Charles Sifford (golfer) and Stevie Wonder (song writer). Stevie Wonder and Mildred Dresselhaus, how cool is that?
on nanotubes are nanometer-sized tubes consisting entirely of carbon. Her contributions to science are many and she is among the most recognized scientists in the field. Dresselhaus worked on graphene and the field of thermoelectrics which has a number of different important applications. How important was her contributions to science and also the promotion of women in science? In 2014 she was awarded the Presidential Medal of Freedom! An award given that year to individuals as diverse as Meryl Streep (actress), Charles Sifford (golfer) and Stevie Wonder (song writer). Stevie Wonder and Mildred Dresselhaus, how cool is that?
Let there be light
Windows! they let us look out on the world from our room and see all sorts of stuff. But could windows do more? Researchers have used nanotechnology to create efficient solar collectors which can collect energy from the sun. They make tiny silicon nanoparticles that are only a few nanometers in size and consists of less than 2000 atoms of silicon. At that size they are not only efficient at collecting solar energy but also don’t impact the ability to see through the windows. Most houses have lots of windows so why not use them not just to look out on the world but generate a bit of energy at the same time.
really, they use a cotton candy machine?
Scientists at Vanderbilt University have discovered a new use for the machine that is used to make cotton candy. Cotton candy is basically sugar that is spun into thin fibers. The cotton candy machine was invented by William Morrison a dentist in collaboration with a candy maker John Wharton. Instead of using sugar, these scientists used a polymer to make a network of thin threads. The threads serve to create tiny channels in gelatin and the polymer is removed leaving just the tiny channels. The channels range in size from a few thousand nanometers to almost 100,000 nanometers. The structures they make are used to study how oxygen and nutrients are transported through tissues by tiny blood vessels. For a video describing the science go to the National Science Foundation
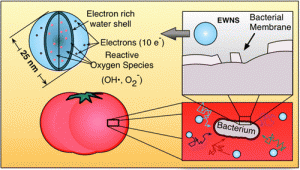
Tiny bubbles
 Tiny bubbles are fun things when you find them in soft drinks where they tickle your nose. Tiny bubble can also be used to clean fruits and vegetables removing bacteria that might cause food-borne illness. Scientists at Virginia Tech University have used cavitation bubbles to scrub the surfaces of tomatoes to remove E. coli and Salmonella. Some day machines to clean these kinds of foods might find their way into your kitchen and help combat food-borne illness.
Tiny bubbles are fun things when you find them in soft drinks where they tickle your nose. Tiny bubble can also be used to clean fruits and vegetables removing bacteria that might cause food-borne illness. Scientists at Virginia Tech University have used cavitation bubbles to scrub the surfaces of tomatoes to remove E. coli and Salmonella. Some day machines to clean these kinds of foods might find their way into your kitchen and help combat food-borne illness.
Happy holidays
To celebrate the holiday season, why not some art? The image is gold nanowires that are  being ‘grown’ on silicon. Nanowires are important for a variety of microelectronics. To grow them scientists have to perfect the recipe by trying different combinations of ingredients, temperatures and other things. These nanowires are about the size of a red blood cell, or about 10,000 nanometers. The gold nanowires were grown by Adnan Korkmaz of the Center for High-rate Nanomanufacturing (CHN) at Northeastern University.
being ‘grown’ on silicon. Nanowires are important for a variety of microelectronics. To grow them scientists have to perfect the recipe by trying different combinations of ingredients, temperatures and other things. These nanowires are about the size of a red blood cell, or about 10,000 nanometers. The gold nanowires were grown by Adnan Korkmaz of the Center for High-rate Nanomanufacturing (CHN) at Northeastern University.
Nanotech Spinach???
OK, sometimes you just have to work with Nature, not against it.
Scientists in California have put specially treated carbon nanotubes, small “threads” of carbon atoms, into spinach plant s ! Why would they want to do this?. Well, scientists have “highjacked” the plant and turned it into a self-powered sensor for EXPLOSIVES. As the plants take up ground water, they also take up any contaminants in the water. The specially treated nanotubes have been treated with a chemical that is sensitive to certain chemicals found in explosives! When the contaminated water and the special nanotubes interact, the plant GLOWS !!! Well, it emits a form of light that is invisible to your eye but can be “seen” by a special camera, like in a smart phone. And they don’t even have to touch the plant, they just have to look at it !!
s ! Why would they want to do this?. Well, scientists have “highjacked” the plant and turned it into a self-powered sensor for EXPLOSIVES. As the plants take up ground water, they also take up any contaminants in the water. The specially treated nanotubes have been treated with a chemical that is sensitive to certain chemicals found in explosives! When the contaminated water and the special nanotubes interact, the plant GLOWS !!! Well, it emits a form of light that is invisible to your eye but can be “seen” by a special camera, like in a smart phone. And they don’t even have to touch the plant, they just have to look at it !!
These particular plants have been engineered to detect explosive residue, but the same technique could be used to make them sense other contaminants, or to sense nutrients or water.
Invisible fish
Fishing is a tricky business because fish are smart. Well maybe not ‘smart’ but they can do things to protect themselves from predators who are trying eat them. Scientists at the University of Texas have discovered tha t the skin of certain fish have tiny nanoscale structures called platelets that reflect polarized light. Polarized light is light that travels in the same plane and that is way light travels through water. By reflecting the polarized light they can appear invisible to a predator and they are most effective when the fish are positioned at an angle similar to the angle at which they are attacked.
t the skin of certain fish have tiny nanoscale structures called platelets that reflect polarized light. Polarized light is light that travels in the same plane and that is way light travels through water. By reflecting the polarized light they can appear invisible to a predator and they are most effective when the fish are positioned at an angle similar to the angle at which they are attacked.
The research was supported by the National Science Foundation.
Nano-bio-wires (or is it bio-nano-wires?)
Sometimes the best solutions come out of unlikely sources. Deep within what most folks would consider muck, scientists have found a way to trick a special kind of bacteria to make tiny wires that are 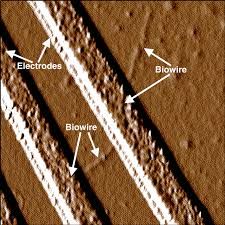 made up of just amino acids. Non-toxic and manufactured using green processes, these nano-bio-wires are only 1.5 nanometers in width. That is much smaller than the best commercial nanofabrication processes can produce. These wires are 2000 times more conducting than just the protein itself leading to a wide number of potential applications. And unlike a lot of other manufacturing processes the process and the products are not toxic. The challenge is to direct the synthesis of these nano-bio-wires to create functional devices.
made up of just amino acids. Non-toxic and manufactured using green processes, these nano-bio-wires are only 1.5 nanometers in width. That is much smaller than the best commercial nanofabrication processes can produce. These wires are 2000 times more conducting than just the protein itself leading to a wide number of potential applications. And unlike a lot of other manufacturing processes the process and the products are not toxic. The challenge is to direct the synthesis of these nano-bio-wires to create functional devices.
Bioinspired materials
Sometimes nature provides the best examples of how to make new nanometer scale materials especially where there is a particular function. Think about how geckos can climb up walls and you can imagine how studying their feet might lead to new adhesives (including things that are going to the mar ket). Difficult scientific challenges are often best figured out using teams and one team at Brandeis University has assembled to study new materials that can move just like living things. One project is focused the shape of cells and how to make artificial materials that move and behave just like cells. These artificial membranes can be used as sensors and also deliver drugs.
ket). Difficult scientific challenges are often best figured out using teams and one team at Brandeis University has assembled to study new materials that can move just like living things. One project is focused the shape of cells and how to make artificial materials that move and behave just like cells. These artificial membranes can be used as sensors and also deliver drugs.
Clean(er) energy
Clean energy is a good thing. We need energy to power a lot of things around us (like cars and iPhones) but we also don’t want to harm the environment by putting things like carbon monoxide into the atmosphere. A number of energy producing devices use catalysts to help carry out chemical reactions. Fuel cells for example take chemical energy and convert it into electrical energy. Scientists at the University of New Mexico and 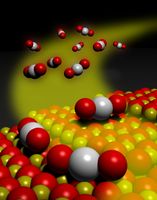 Washington State University have collaborated to develop a new kind of platinum catalyst to convert carbon monooxide (that invisible gas that can kill you) into carbon dioxide. Platinum is an expensive metal but also a very good catalyst. These scientists created a nanometer-scale material that traps platinum better and makes it a more efficient catalyst. The key was using something called cerium oxide to trap the platinum and keep it from forming aggregates which wasn’t good for catalysis. They hope to take these research discoveries and translate them into practical processes for making cleaner energy.
Washington State University have collaborated to develop a new kind of platinum catalyst to convert carbon monooxide (that invisible gas that can kill you) into carbon dioxide. Platinum is an expensive metal but also a very good catalyst. These scientists created a nanometer-scale material that traps platinum better and makes it a more efficient catalyst. The key was using something called cerium oxide to trap the platinum and keep it from forming aggregates which wasn’t good for catalysis. They hope to take these research discoveries and translate them into practical processes for making cleaner energy.
Drink like a butterfly
Scientists often use things in nature to design nanometer-scale tools. Things in nature are the result of years (and years) of evolution  providing scientists with a final design that has undergone a lot of testing and refinement. The butterfly proboscis is an example, it is a long tube that butterflies use to suck up liquids. Researchers at Clemson University have studied the butterfly proboscis and used that to engineer a nano-sipper. They think it could be used to suck up and dispense very tiny (nanoliters, or one-billionth of a liter—think of a 2 liter bottle of soda—there are about 50,000 nanoliters in a drop). The advantage of these butterfly inspired straws are that they don’t get clogged which is a real problem with nanometer-scale fluid channels
providing scientists with a final design that has undergone a lot of testing and refinement. The butterfly proboscis is an example, it is a long tube that butterflies use to suck up liquids. Researchers at Clemson University have studied the butterfly proboscis and used that to engineer a nano-sipper. They think it could be used to suck up and dispense very tiny (nanoliters, or one-billionth of a liter—think of a 2 liter bottle of soda—there are about 50,000 nanoliters in a drop). The advantage of these butterfly inspired straws are that they don’t get clogged which is a real problem with nanometer-scale fluid channels
Nano and the dentist
Nanotechnology can be used to create new materials with superior properties. Solar cells, cancer drugs and now the visit to the dentist office could be a bit better (well still no fun!). New dental materials are being created. “These resin-based composites (RBCs) containing nanopart icles exhibit a high surface free-energy that exerts differential behavior in terms of mechanical and physicochemical properties, such as an excellent color density, low polymerization shrinkage, adequate surface brightness, low surface roughness, resistance to fracture, and excellent adherence to dental tissues,” wrote the authors. Wow that is a mouthful. Translation? The stuff that they use to repair cavities and damaged teeth is going to be better, smoother and more like real teeth.
icles exhibit a high surface free-energy that exerts differential behavior in terms of mechanical and physicochemical properties, such as an excellent color density, low polymerization shrinkage, adequate surface brightness, low surface roughness, resistance to fracture, and excellent adherence to dental tissues,” wrote the authors. Wow that is a mouthful. Translation? The stuff that they use to repair cavities and damaged teeth is going to be better, smoother and more like real teeth.
Award winning nano-pix
Each year the National Nanotechnology Coordination Office (a friend of Nanooze) hosts a contest for the best nano images. This years winner was taken by Elizabeth Sawicki from the University of Illinois. The images are of nanometer-sized gold nanoparticles in the brain after being whiffed up the nose. (not people, rats). The work is being done to develop new treatments for things like strokes. Ms. Sawicki is in the Medical Scholars Program. Congrats!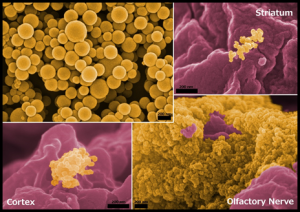
National Science Foundation supports nano sites across the US
The National Science Foundation has awarded approximately $81M over the next five years to support a total of 16 different research sites to serve nanotechnologists across the US and the world. The National Nanotechnology Coordinated Infrastructure will give researchers the tools to make a bunch of new nano things. The 16 different sites involve 27 universities from the East to the West coast. One of the sites at Cornell University will also support Nanooze! That is a great reason to smile!
New Gecko grippers
Scientists at the University of Pennsylvania have developed a new nano-gripper that is based upon a gecko’s foot. Gecko’s are able to climb walls because their feet are covered with tiny nanostructures that stick to all sorts of surfaces. Think Spiderman. These new nano-grippers were made tunable so that they might stick and release from different surfaces. They are made of a hard plastic core and surrounded by a softer silicon rubber. The work was supported by the National Science Foundation
Nanotechnology and your Teeth
Your mouth is FILTHY !! Really ! Your mouth is full of germs, which is why you are supposed to brush your teeth well. As you get older, and particularly if you don’t keep your teeth clean, bacteria (germs!) get down under your gums and make them red and swollen. That may even make your teeth fall out !
Good oral hygiene (that’s a fancy word for brushing your teeth well) can help. And dentists can use antibiotics (like penicillin) to kill the germs if they get bad. But sometimes those germs become resistant to antibiotics. Then what are you supposed to do !!
Well, maybe nanotechnology can come to the rescue, in particular, a material called graphene oxide. Graphene (graf-ene) is one of the new wonder materials of nanotechnology. Graphene is a form of carbon; in graphene , the carbon atoms are bonded in a large sheet that is only ONE ATOM THINK. On a microscopic scale, graphene would look like “chicken wire” with a carbon atom at every junction. The fact that it is only one atom thick gives graphene some very interesting properties. When these single atomic sheets react with oxygen, you can get Graphene Oxide, which also has some pretty cool properties. For one, it dissolves in water!
Scientist have recently determined that graphene oxide can kill some of the stubborn bacteria in your mouth!! Scientists are still studying whether this is practical in the real world, but maybe, someday, you will have a nanotechnolgy mouthwash containing graphene oxide nanoparticles !!
How they do it…chameleons
Chameleons change their color to hide from the bad guys  but also to fight off other chameleons for their territory. How do they do that? New research suggests they can quickly change tiny nanocrystals made out of guanine. One layer in their skin cells can change spacing quickly resulting in color changes. A small change in spacing can cause a big change in their apparent color
but also to fight off other chameleons for their territory. How do they do that? New research suggests they can quickly change tiny nanocrystals made out of guanine. One layer in their skin cells can change spacing quickly resulting in color changes. A small change in spacing can cause a big change in their apparent color
Nanobombs kill food-borne pathogens
At some time or another (or another, and another) we get the dreaded food-borne illness. Stomach aches and yes diarrhea. There are many different culprits from bacteria to viruses to parasites. Scientists have developed a way of killing some of these agents on foods using nothing more than super-charged water. The process uses water nanodroplets that pass through an electric field and become charged. These charged water nanodroplets then are able to help kill bacteria. The team tested the system with some food-borne illness causing agents on both foods and cutting boards.