The four important things to know about nanotechnology
So nanotechnology is new and cool and there are zillions of things to read about it. But what is really important to know before trying to figure out what all this nanotechnology stuff is about? Well first…
Nanotechnology is all about making things that are less that 100 nanometers in size. That is pretty small, we are talking about atomic sized stuff.
So here are four things to keep in mind.
All things are made of atoms. Really? Everything? Not exactly but most stuff, like you, your dog, your toothbrush, even your computer. Things like light, sound and electricity aren’t made of atoms, but the sun and the moon are all made of atoms. That is a lot of atoms. Even when there seems to be nothing, there are lots of atoms. One cubic centimeter of air (that is the size of a sugar cube) has around 1019 atoms ( 10,000,000,000,000,000,000 atoms). There are around 109 people (1,000,000,000 people) (actually about 7 billion, but we are talking round numbers–when doing rough comparisons, scientists often talk in round numbers, we call it “orders of magnitude”–it helps us see the “big” picture) on the Earth so we are off more than a billion times. If there are that many atoms in some thin air, well there must be atoms everywhere.
At the nanometer scale atoms are in constant motion. So how come we just don’t shake apart. Because the motion is atomic motion and when you have a lot of atoms the motion of any individual atom is hard to see. Moving atoms are hard to see them, so to see atoms, scientists do a couple of things. First they pull a giant vacuum on whatever they want to look at just to get rid of all of the other atoms that might crud up their atoms. Remember that there are around 1019 molecules in one cubic centimeter of air. And scientists can’t really tell one atom from another when they are trying to see them. Next, they need to cool things down to slow the atoms down as much as possible. Cold, almost absolute zero which is about -460°F. You can’t get to absolute zero but you can get close.
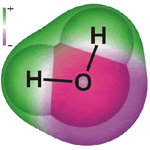 Molecules have size and shape. Atoms bond together to form molecules. Water is a molecule made up of one oxygen atom and two hydrogen atoms, so it is called H2O. It has a particular shape because of the angle of the bonds that hold the two hydrogen atoms to the oxygen. That gives it a shape in 3D.
Molecules have size and shape. Atoms bond together to form molecules. Water is a molecule made up of one oxygen atom and two hydrogen atoms, so it is called H2O. It has a particular shape because of the angle of the bonds that hold the two hydrogen atoms to the oxygen. That gives it a shape in 3D.
Bigger molecules have more complicated shapes. Like the protein on the right, insulin, which helps us store and use energy from food. Its shape helps insulin to function. The shape of insulin is a result of all of the atoms in insulin and all of the bonds and all of the interactions that happen between all of those atoms and all of those bonds. The shape of molecules is pretty complicated.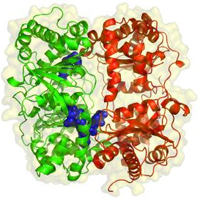
Molecules in the nanometer scale environment have unexpected properties. What is cool about nanotechnology is that we can make things that don’t behave like we expect. So we can make these tiny little particles called quantum dots that are made of just a few hundred atoms. They fluoresce, which means that if you shine a light of a certain color on them, they give off light at a different color (wavelength). Their properties depend on their size, which is only a few nanometers, and the atoms that they are made up of. Quantum dots have applications in things like solar cells and detecting diseases.
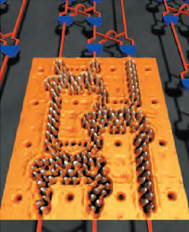
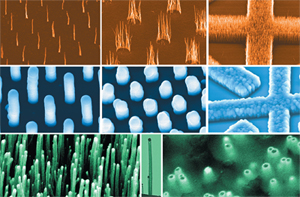 Another example is carbon nanotubes, which are long tubes of just carbon (shown on the left), but carbon which is bonded in a very special way. Because of the way carbon atoms are bonded together in carbon nanotubes they have unique properties. One is that they can act as semiconductors, meaning that sometimes they allow electrons to pass through and other times they don’t. Semiconductors are really important for making computer chips since they can be made to act like little switches turning on and off depending upon if they are allowing electrons to move through or not. Carbon nanotubes are also really strong. Stronger than steel. So one application is to create a space elevator where you could get on and get pulled into space. For now that is way in the future, but carbon nanotubes are being used in tennis rackets and bicycles where they make them strong without making them heavy.
Another example is carbon nanotubes, which are long tubes of just carbon (shown on the left), but carbon which is bonded in a very special way. Because of the way carbon atoms are bonded together in carbon nanotubes they have unique properties. One is that they can act as semiconductors, meaning that sometimes they allow electrons to pass through and other times they don’t. Semiconductors are really important for making computer chips since they can be made to act like little switches turning on and off depending upon if they are allowing electrons to move through or not. Carbon nanotubes are also really strong. Stronger than steel. So one application is to create a space elevator where you could get on and get pulled into space. For now that is way in the future, but carbon nanotubes are being used in tennis rackets and bicycles where they make them strong without making them heavy.
Image Source: Nasa | University of Chicago